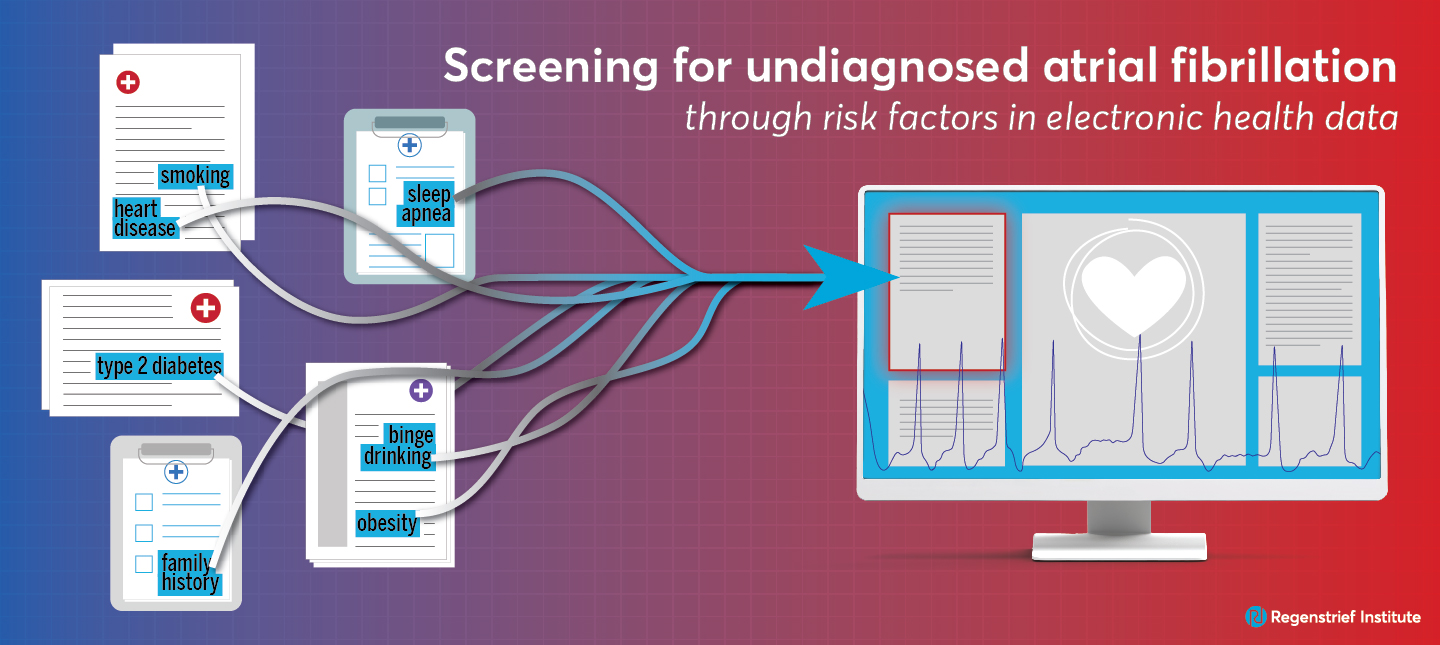
Non-invasive, inexpensive approach provides a practical option for proactive screening
INDIANAPOLIS – AFib (short for atrial fibrillation), a common heart rhythm disorder in adults, can have disastrous consequences including life-threatening blood clots and stroke if left undetected or untreated. A new study demonstrates that UNAFIED, a highly accurate artificial intelligence (AI) prediction model which uses machine learning to parse information acquired from a patient’s electronic health record (EHR) to predict whether a patient has or might develop detectable AFib within the following two years, can be easily integrated into the healthcare workflow.
UNAFIED is an acronym for Undiagnosed Atrial Fibrillation prediction using Electronic health Data.
Testing implementation and performance in real world conditions, the researchers reported that physicians in a busy medical practice in the Eskenazi Health system in Indianapolis who regularly used the UNAFIED risk prediction model found it easy to use and not time consuming. Most significantly, physicians participating in the study indicated that they believed it helped improve patient care. The non-invasive, inexpensive approach provides a practical option for proactive screening of patients, especially the large number of individuals at elevated risk for AFib.
Individuals at higher risk for AFib include adults living with obesity, many types of heart disease, Type 2 diabetes or sleep apnea, as well those who are smokers or binge alcohol drinkers or have a family history of the disease.
“Unfortunately, atrial fibrillation can be silent until it’s disastrous. We developed and validated this risk prediction model to find the instances where atrial fibrillation was silent but still occurring or likely to occur,” said Regenstrief Institute Research Scientist Randall Grout, M.D., M.S. “The primary goals of the UNAFIED model are preventing very significant negative medical outcomes and even death.
“Using such indicators as sex, height and weight, prior diagnoses of heart or kidney disease — information already easily available to the clinician — our model performed at the leading edge. It doesn’t require extra steps, making it easy for clinicians to integrate into their practice.”
Dr. Grout is the first author of the UNAFIED clinical implementation study, a co-author of the national validation study and the first author of the development study. In addition to his Regenstrief appointment, Dr. Grout is a faculty member of the Indiana University School of Medicine and chief health informatics officer at Eskenazi Health.
In the study of clinical implementation, UNAFIED was integrated into the EHR system of a busy cardiology clinic, enabling the algorithm upon which UNAFIED is based to calculate the predicted risk for each patient individually. If the risk factor was found to be above a certain threshold, the model provided visual indicators to the cardiologist that the patient might have an elevated risk of undetected AFib or of developing AFib within the next two years. The workflow also provides recommendations such as performing follow-up heart rhythm and other testing as well as presenting ways to document within the EHR for higher risk or that a patient may actually be experiencing AFib, even if the condition had been previously ruled out. Respectful of professional expertise and experience, the model offers the physician the option of overriding or bypassing the prompts.
According to the Centers for Disease Control and Prevention, there are more than 454,000 hospitalizations with AFib as the primary diagnosis in the U.S. annually. The condition contributes to an estimated 158,000 deaths in the U.S. each year.
Dr. Grout notes that while the algorithm on which UNAFIED is based was built to predict undetected AFib, lessons learned from the development of this model could be employed to develop algorithms for models focused on other conditions as well as specific populations or geographic areas. While some of the predictor variables used may be the same in many or most models – for instance age of patient – others, such as a history of a certain diagnoses, could be customized for the specific disease under scrutiny.
Clinical implementation study
“Screening for undiagnosed atrial fibrillation using an electronic health record‒based clinical prediction model: clinical pilot implementation initiative” is published in BMC Medical Informatics and Decision Making. It was supported by Pfizer Inc.
All authors and affiliations as listed in the publication:
Randall W Grout1,2,3, Mohammad Ateya4, Baely DiRenzo5, Sara Hart4, Chase King6, Joshua Rajkumar7, Susan Sporrer4, Asad Torabi6, Todd A Walroth5, Richard J Kovacs6,5.
1Indiana University School of Medicine, Indianapolis, IN, USA.
2Eskenazi Health, Indianapolis, IN, USA.
3Regenstrief Institute, Indianapolis, IN, USA.
4Pfizer Inc, New York, NY, USA.
5Eskenazi Health, Indianapolis, IN, USA.
6Indiana University School of Medicine, Indianapolis, IN, USA.
7Franciscan Physician Network – Indiana Heart Physicians, Indianapolis, IN, USA.
National validation study
“Validation, bias assessment, and optimization of the UNAFIED 2-year risk prediction model for undiagnosed atrial fibrillation using national electronic health data” is published in Heart Rhythm. The study was supported by Pfizer Inc.
All authors and affiliations as in the publication:
Mohammad Ateya1, Danai Aristeridou1, George H Sands1, Jessica Zielinski1, Randall W Grout2,3, A Carmine Colavecchia1, Oussama Wazni4, Saira N Haque1.
1Pfizer Inc, New York, New York.
2Regenstrief Institute, Indianapolis, Indiana.
3Indiana University School of Medicine, Indianapolis, Indiana.
4Cleveland Clinic, Cleveland, Ohio.
Development study
“Development, validation, and proof-of-concept implementation of a two-year risk prediction model for undiagnosed atrial fibrillation using common electronic health data (UNAFIED)”
This initial study utilized regional data from the Indiana Network for Patient Care (INPC), which was developed by the Regenstrief Institute and is operated by the Indiana Health Information Exchange.is The work published in BMC Medical Informatics and Decision Making. It was supported by the Agency for Healthcare Research and Quality K12 HS026390 (PI: Randall Grout, M.D., M.S.)
All authors and affiliations as listed in the publication:
Randall W Grout1,2, Siu L Hui3,4,5, Timothy D Imler3,6, Sarah El-Azab4, Jarod Baker3, George H Sands7, Mohammad Ateya7, Francis Pike5.
1Center for Biomedical Informatics, Regenstrief Institute, 1101 W Tenth St, Indianapolis, IN, 46202, USA.
2Department of Pediatrics, Indiana University School of Medicine, Indianapolis, IN, USA.
3Center for Biomedical Informatics, Regenstrief Institute, 1101 W Tenth St, Indianapolis, IN, 46202, USA.
4Research Services, Regenstrief Institute, Indianapolis, IN, USA.
5Department of Biostatistics, Indiana University School of Medicine, Indianapolis, IN, USA.
6Division of Gastroenterology and Hepatology, Indiana University School of Medicine, Indianapolis, IN, USA.
7Pfizer Inc, US Medical Affairs, New York, NY, USA.
Randall Grout, M.D., M.S.
In addition to his role as a research scientist with the Clem McDonald Center for Biomedical Informatics at Regenstrief Institute, Randall Grout, M.D., M.S., is the Chief Health Informatics Officer at Eskenazi Health. He is also an assistant professor of pediatrics at the Indiana University School of Medicine.