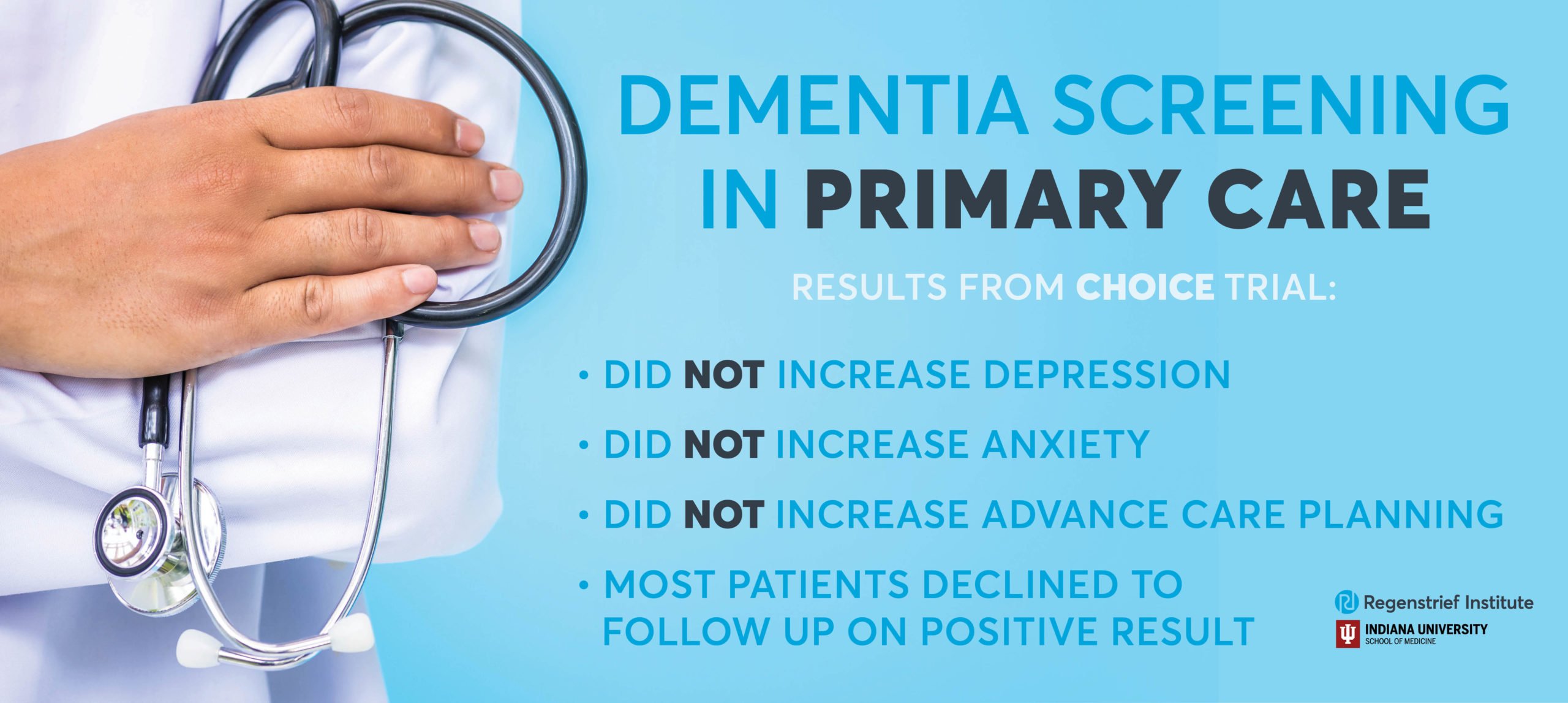
Dementia screening did not induce depression or anxiety in older adults
Research scientists at Regenstrief Institute and Indiana University School of Medicine have conducted the first randomized controlled trial to evaluate the pros and cons of population screening for dementia. The researchers found no harm, as measured by patient reported depressive and anxiety symptoms, from screening for Alzheimer’s disease and related dementia in diverse rural, suburban and urban primary care clinics in Indiana.
Furthermore, the trial did not identify any benefit from screening in reducing emergency department visits and hospitalizations, or increasing advance care planning.
“Many patients and families have concerns that dementia screenings may create anxiety or depression in patients because there is, as yet, no cure for this disease. However, this study shows that is not the case,” said Nicole Fowler, PhD, MHSA, associate director of the Indiana University Center for Aging Research at Regenstrief Institute.
Dr. Fowler, an assistant professor of medicine at IU School of Medicine and a Regenstrief research scientist, is first author of the new study, which is published in the Journal of the American Geriatrics Society.
“Dementia screening provides awareness for the patient and their family, allowing them to take action – including advance care planning — and we now know that the screening does not harm the patient. Though we found many patients declined to follow up a positive screening, the knowledge obtained from a screening at least allows them to enter a watchful waiting period or choose to be engaged,” Dr. Fowler said.
While 70 percent of study participants who screened positive for cognitive impairment declined a follow up diagnostic assessment, those who did complete a follow up and then received collaborative care had significantly decreased hospital admissions as compared with study participants who were not screened but later developed cognitive impairment. Previous studies led by IU School of Medicine and Regenstrief research scientists have found that the collaborative dementia care model decreased behavioral and psychological symptoms in patients living with dementia and reduced healthcare utilization, resulting in annual cost savings ranging from $908 to $2,856 per patient.
“For a number of reasons, including the lack of drugs to treat dementia and the stigma around the condition, people are hesitant to engage in the next steps of the process after screening,” said Dr. Fowler. “The health care system needs to help bridge this gap and encourage people to follow up on the results of screening tests as they would for any other condition.”
The study noted that “the finding of statistical equivalence of screening on patients’ symptoms of depression and anxiety” is important given that previous studies measuring the public’s perceived attitude of dementia screening reported that patients were concerned that this screening would make them feel depressed or anxious. In fact, it did not.
Dementia affects more than 5 million people in the United States and is frequently unrecognized and underdiagnosed in primary care settings, where most older adults receive their health care. It is estimated that as many as half of primary care physicians are unaware of their older patients’ cognitive status.
More than 4,000 primary care patients age 65 years and older were enrolled in the randomized, controlled Indiana University Cognitive Health Outcomes Investigation of the Comparative Effectiveness of Dementia Screening (CHOICE) trial. Two-thirds of study participants were female; two-thirds of study participants were white.
“This study is groundbreaking because we have scientifically negated the concern that dementia screening may be harmful,” said Malaz Boustani, MD, MPH, the study’s senior author and principal investigator of the trial. “Until now, the lack of evidence of potential harm of dementia screening has been a barrier to dementia screening in primary care. Hopefully our finding of no harm from screening has eliminated this obstacle.”
Additional authors on the paper are Greg Sachs, M.D., a Regenstrief Institute research scientist, professor of medicine and division chief of General Internal Medicine and Geriatrics at IU School of Medicine; and Anthony J. Perkins, M.S., and Sujuan Gao, PhD, IU School of Medicine.
This study was funded by National Institute on Aging grant R01AG040220.
Dr. Fowler is currently leading the Caregiver Outcomes of Alzheimer’s Disease Screening (COADS) trial examining the impact of dementia screening of older adults on their family member caregivers.
About IU School of Medicine
IU School of Medicine is the largest medical school in the U.S. and is annually ranked among the top medical schools in the nation by U.S. News & World Report. The school offers high-quality medical education, access to leading medical research and rich campus life in nine Indiana cities, including rural and urban locations consistently recognized for livability.
About Regenstrief Institute
Founded in 1969 in Indianapolis, the Regenstrief Institute is a local, national and global leader dedicated to a world where better information empowers people to end disease and realize true health. A key research partner to Indiana University, Regenstrief and its researchers are responsible for a growing number of major healthcare innovations and studies. Examples range from the development of global health information technology standards that enable the use and interoperability of electronic health records to improving patient-physician communications, to creating models of care that inform practice and improve the lives of patients around the globe.
Regenstrief Institute is celebrating 50 years of healthcare innovation. Sam Regenstrief, a successful entrepreneur from Connersville, Indiana, founded the institute with the goal of making healthcare more efficient and accessible for everyone. His vision continues to guide the institute’s research mission.
About Nicole Fowler, PhD, MHSA
In addition to her role as a Regenstrief Institute research scientist and associate director of the Center for Aging Research, Dr. Fowler is an assistant professor at Indiana University School of Medicine and an implementation scientist at the Center for Health Innovation and Implementation Science.
About Malaz Boustani, MD, MPH
Malaz Boustani, M.D., MPH, is a Regenstrief Institute research scientist, the founding director of the Center for Health Innovation and Implementation Science, and holds the Richard M. Fairbanks Chair in Aging Research at Indiana University School of Medicine. Dr. Boustani is also the founding director and chief innovation and implementation officer at the Sandra Eskenazi Center for Brain Care Innovation, in addition to serving as director of senior care innovation at Eskenazi Health.